
Know How Auditing Suppliers Help to Grow Your Business
Maintaining supplier relationships has become more critical than ever. Though an organization depends on suppliers for the production, it doesn’t mean that all the flaws in the supplier processes should be ignored. There should be stringent processes...

Using Quality Metrics to Improve QMS Strategy and Processes
Most of the leading organizations have realized the fact that business success depends only on happy and satisfied customers. And, customer satisfaction can be achieved only with Quality. it has become insanely significant to encourage a culture of q...

Effective Communication's Role in Change Management Process
If you want to make the process of change implementation smooth, put fewer efforts, and assist your employees, you must establish a streamlined process that makes the change management easy without facing any resistance. As we all know that the busin...

Nonconformance VS Noncompliance: A Brief Guide
Do you often feel confused with the usage of terms Nonconformance and Noncompliance? If yes, you are not alone. Since both the terms are used interchangeably, the confusion is the common outcome. In this post, we will understand the differences betwe...

Learn how employee training impacts workplace productivity
Modern organizations that want to get maximum returns from the investments need to focus on creating a human capital difference through effective training and development programs for their employeesfor better Workplace Productivity. As they say, the...

Product-Centric Quality Approach for Improved CAPA Processes
Designing a product that serves its intended purpose is critical for medical device manufacturers, as it could have a great impact on a person’s health- for that matter even life. You can still manage your tasks if your internet modem is not working ...

9 Significant Reasons You Need eQMS instead of QMS
In the era of digitization, every industry is experiencing transformations more than they ever expected including the highly regulated industries including manufacturing, healthcare and lifesciences. It cannot be denied that such industries need to a...

What is Closed Loop Quality Management System?
In the quality domain, it’s a common belief that sooner a nonconformance gets identified and resolved, lesser will be its impact. The advent of technology and cloud-based solution for quality management have made this belief even more actionable. The...

What is Data Quality Management? Why is it important?
With the increasing popularity of analytics and big data, business owners are becoming aware of the significant role that data management plays for an organization. Credit goes to factors like forecasting customer expectations, product management, av...

Five Common CAPA Mistakes Companies Need to Avoid
CAPA is often the most discussed topic among all the aspects of the closed-loop quality management system due to the critical role it plays. Still, companies face difficulty in implementing it right and feel frightened by the thoughts of regulatory a...

Address Quality & Manufacturing Challenges in Life Sciences
Lifesciences industry is undergoing a huge digital transformation wherein they are adopting newer and smarter technologies for attaining operational excellence. The best thing about the digital transformation is the evolution of quality management so...

9 Tips for an Effective Supplier Strategy for Combo Products
With the evolution of the combination products developed by medical device manufacturers and pharmaceutical companies in partnership, the need for a robust quality management system is accelerated. In an effort to improve health and safety for the pa...

Change Management: Mastering the Art of Subtle Transitions
The ever-growing and evolving business world, an organization needs to cope with the technological advancements and changes to be successful. More you will be your adaptability; higher will be the chances of success. Hence, managing change has become...

Electronic Signatures: Modern Quality Document Verification
As organizations are going paperless with the adoption of electronic document management systems and record-keeping, the next natural evolution for them is electronic signatures. The norm of digital signatures has offered companies a secure way to ve...

What are the Challenges in Managing Medical Device Records
The medical device industry has witnessed tremendous growth in the past 10-20 years – in terms of revenues as well as the technical complexities of the product. There have been technological evolutions too in the medical device industry, but healthca...

7 Reasons: It’s Time for Cloud-Driven QMS
Organizations need effective EQMS to manage their quality management processes efficiently while mitigating compliance and operative risks. A quality management solution ensures you have streamlined processes to maintain a balanced structure and achi...

Quality Management Vs Quality Control
Quality is, indeed, a broader aspect to understand than what it sounds. Quality Assurance, Quality Control, and Quality Management are commonly used terminologies by organizations with respect to the quality of their products and services. However, i...

Know How Quality Management and Risk Management Inter-related
The organizations today are taking steps for continuous improvement of quality in their products and services to meet customer requirements and compliance standards simultaneously. The focus on continual improvement helps them to drive operational ex...

Proactive vs Reactive Quality: Which Approach is Better
Building a culture of quality depends on reactive and proactive functions, processes, and workflows in the organization. However, the best approach to attain quality has always been debatable. In this post, we will look at the pros and cons of both r...

Drive Continuous Improvement by Analyzing Audit Findings
Creating an environment of continuous improvement requires a management-driven mindset and detailed analysis of audit findings that should be conveyed at all the levels in the organization. Thanks to the advanced audit management software, it has bec...

Industry 4.0: A Quick Brief on Benefits and Challenges
Industry 4.0 indicates the start of a new industrial revolution to enhance communication, analysis, and utilization of the resources in the manufacturing companies by bringing together two powerful components: advanced techniques of manufacturing and...

Effectively Managing Automotive Quality through Qualityze EQMS
Because of its complex supply chain dependencies, societal implications, widespread use of social media and its ability to impact product quality issue: even a single misstep or oversight in quality can cause massive setbacks within a matter of minut...

EQMS Way of Managing Your Training Requirements
Employee training has always been a vital component of a company’s quality culture and actually solidifies it but in certain scenarios EQMS is the best choice to effectively manage the training requirements in an organization. With the added benefits...
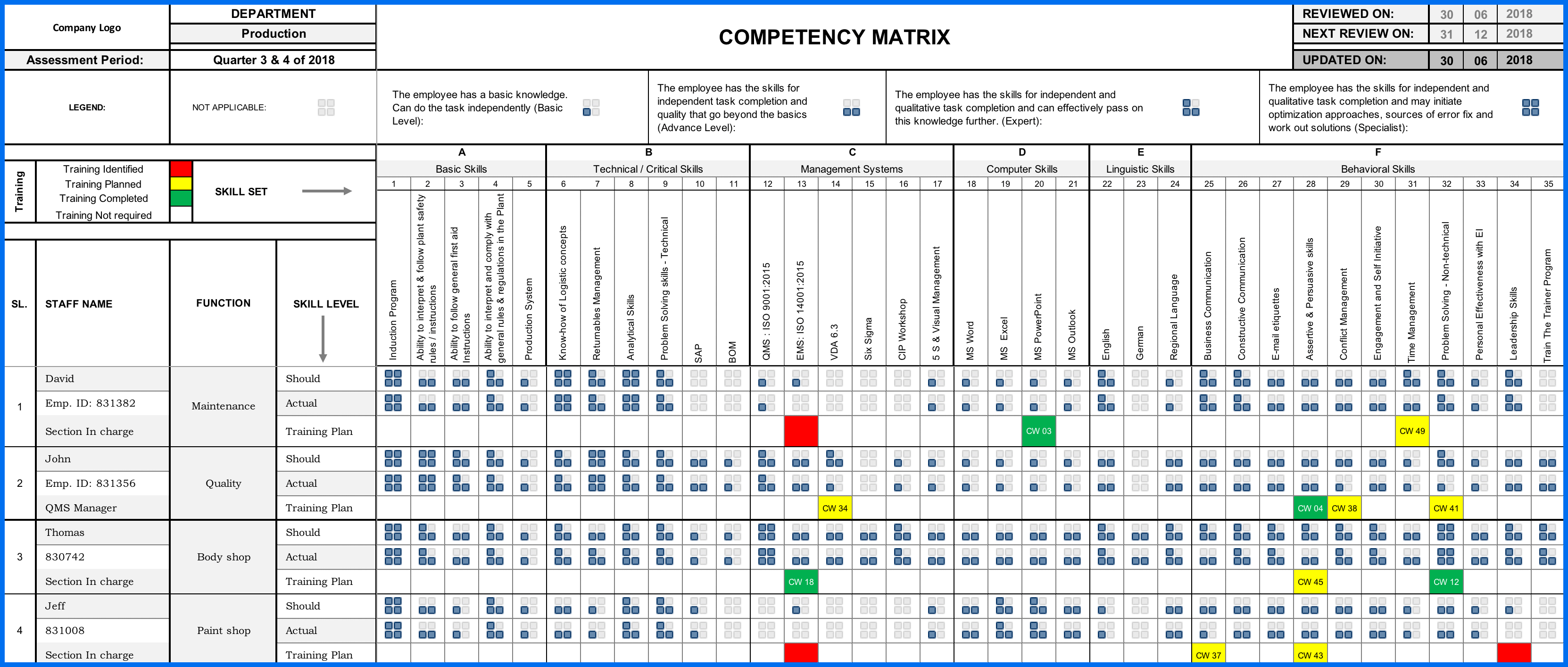
How to Build an Effective Competency Matrix or Skills Matrix
In ISO 9001:2015, the clause 7.2 specifically requires an organization to determine the necessary competency matrix or skills matrix of employees and ensure they are qualified on the basis of appropriate education, training, or experience. Therefore,...

Employee Competence in ISO 9001:2015
This blog refers to ISO 9001:2015 Clause 7.2 “Competence” and is specifically relevant to organizations seeking to implement or update to the latest ISO 9001:2015 version -. At the heart of creating Excellence in People is building a high-performance...

ISO 9001:2015 - Revealing Perspectives of Outsourced Process
This blog aims to help organizations understand the meaning and interpret the requirements stated in ISO 9001:2015 concerning “outsourced process” and how to carry out a meaningful gap analysis against your current processes and procedures. The Claus...

After an Audit, What Next?
Have you at any point felt on edge about something that was going to occur at your working environment.? Imagine if the “something” was called an audit, increasing your anxiety to a stratospheric level regardless of the preparations you have made for...

Requirement of Knowledge Management in ISO 9001:2015
This blog refers to ISO 9001:2015 Clause 7.1.6 “Organizational knowledge” and is specifically in relevance to organizations seeking to implement or update to the latest version of ISO 9001:2015 along with anyone thinking about implementing the intern...

What to think for Risk-Based-Thinking (RBT) in ISO 9001:2015?
The ISO standards are reviewed every five years and accordingly revised if needed. This is mainly to keep pace with changing business environment and to provide effective tools to tackle new industry challenges specifically in Lifesciences and manufa...

Not everything needs CAPA management process
Oftentimes in business it is felt that in the quest of excellence organizations tend to capture all adverse effects (events) to initiate CAPA Management Process by overlooking the question of its criticality. Surprisingly it happens in both type of o...





























