Change Management
Navigate Change with Confidence
As the digital era continues to reshape every facet of business, both regulatory bodies and organizations find themselves compelled to not only keep up but also to thrive amidst this relentless transformation. What was once considered conventional and sufficient now pales in comparison to the demands posed by the complexities of this new landscape.
Adapting to the Winds of Change for Unparalleled Operational Effectiveness
This is precisely where comprehensive Change Management solution of Qualityze steps in, emerging as an imperative that no modern entity can afford to overlook. With Qualityze you will have a standardized process, to adapt to changes while maintaining operational efficiency. Our software serves as a valuable tool for effectively managing changes to documents, products, or process, and effectively navigating the challenges that come with change. With Qualityze as a guiding light, illuminating the path towards a future you can see the change as an opportunity to be harnessed rather than a challenge to be overcome.

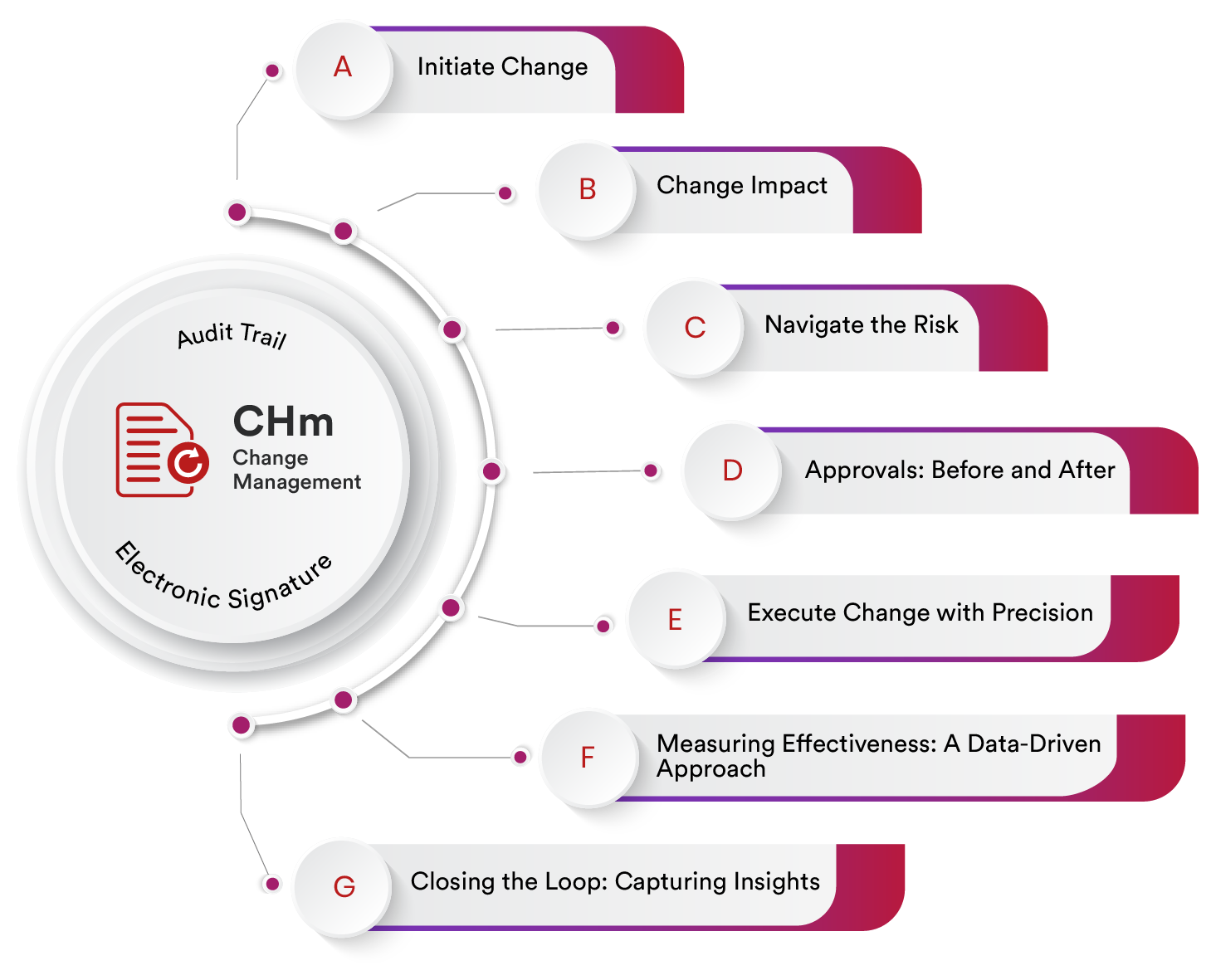
Initiate Change
Change begins with recognizing the need for it. Qualityze Change Management starts by facilitating a meticulous process of initiation. This stage involves identifying the areas where change is required and thoroughly evaluating the reasons behind it. By doing so, organizations ensure that every change effort is purposeful, aligned with overarching goals, and equipped to deliver tangible benefits.

Change Impact
Change is more than a singular event; it's a series of ripples that can potentially affect every facet of an organization. With Qualityze, you can perform a comprehensive change impact analysis that offers a holistic view of these potential effects. Collaboration feature enable you to assess the interconnected systems, critical processes, and key stakeholders to make an informed decision. This empowers your team to implement changes in a way that minimizes disruptions and maximizes positive outcomes.

Navigate the Risk
Change and risk often go hand in hand. Qualityze understands this, which is why it features an integrated risk assessment and mitigation module. As change unfolds, risks naturally emerge. Using Qualityze, you can identify and assess these risks in realtime. What's more, it assists in devising and implementing appropriate mitigation strategies. The goal is simple yet profound: to minimize negative impacts and disruptions, ensuring that the winds of change remain favorable.

Approvals: Before and After
Navigating change often involves obtaining approvals at critical junctures. Qualityze Change Management streamlines this process by facilitating pre- and post-change approvals. Before implementation, all necessary authorizations are secured, ensuring that change adheres to regulations and policies from the outset. Similarly, after changes have been executed, post-implementation approvals validate that the intended outcomes have been achieved and that any required adjustments have been made.

Execute Change with Precision
The implementation phase is where the blueprint becomes reality. Qualityze excels in executing approved changes systematically. By following a structured approach, disruptions are minimized, and efficiency is maximized. This precision ensures that the wheels of operations keep turning smoothly even amidst change, safeguarding the continuity of business processes.

Measuring Effectiveness: A Data-Driven Approach
Change is not an end in itself; it's a means to achieve specific outcomes. Qualityze recognizes this and introduces an effectiveness review stage. Post-implementation, a Qualityze allows you to perform a thorough evaluation to measure how well the changes have delivered their intended results. This data-driven approach provides insights into the effectiveness of the change management process and highlights areas for potential improvement.

Closing the Loop: Capturing Insights
Every change is a learning opportunity. Qualityze Change Management acknowledges this by including a closure stage. Here, change requests are formally closed, and the process is documented. More importantly, lessons learned are captured – insights that can inform future change initiatives. This continuous learning cycle ensures that the organization becomes more adept at managing change with each iteration.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
Qualityze Change Management enables organizations to significantly reduce the Cost of Quality related to change processes. With our solution, you can streamline change management, mitigate risks, and enhance overall quality.
Here's how Qualityze Change Management helps you save on the Cost of Quality:
- Minimizes the cost of rework and retesting by ensuring thorough impact analysis before implementing changes.
- Reduces the cost of compliance by maintaining proper documentation and audit trails for change activities.
- Improves efficiency by automating change workflows and approvals, eliminating manual errors and delays.
- Enhances communication and collaboration among stakeholders, preventing misunderstandings and costly mistakes.
- Facilitates effective risk management by identifying potential impacts of changes and implementing preventive measures.
- Provides real-time visibility into change processes, enabling proactive decision-making and cost control.
Recognitions



Change Management benefits to various operational functions
Every business function has unique expectations from a solution, find out what Qualityze can deliver from your functions perspective.
Quality Assurance
The role of Change Management in Quality Assurance is to effectively manage and control changes within an organization's processes, systems, and products to ensure compliance with quality standards and minimize disruptions.
Risk Management
Change management provides structured processes and strategies to identify, assess, and mitigate risks associated with organizational changes, ensuring successful implementation and minimizing negative impacts.
Process Improvement
Change Management plays a crucial role in process improvement by effectively managing and implementing changes to ensure smooth transitions and successful adoption of improved processes.
Supply Chain Management
Change Management streamlines Supply Chain Management by ensuring smooth transitions, minimizing disruptions, and effectively implementing changes to optimize processes and enhance operational efficiency.
Production Management
Change Management in Production Management plays a crucial role in ensuring smooth transitions, minimizing disruptions, and effectively implementing changes to optimize production processes and maintain operational efficiency.
Compliance and Regulatory Affairs
Change Management in Compliance and Regulatory Affairs ensures that organizations effectively implement and adapt to regulatory changes, minimizing risks, ensuring compliance, and maintaining operational integrity.
Experience Qualityze Difference in managing Quality
We empower organizations to uphold exceptional quality standards while providing the following advantages:
Configurable Cloud-Based Platform
Our system is developed on the world's foremost cloud-based platform, Salesforce.com, which allows businesses to effortlessly personalize it to their exact requirements. It is an adaptable and scalable system that expands in tandem with the growth of your organization. Its wide-ranging configurability broadens the functional scope of an EQMS to an infinite array of creative applications, making it an extremely valuable solution for your organization.
Compliance Ready
Our system adheres to compliance standards for every industry and follows the relevant regulations set by regulatory committees, such as the Food and Drug Administration (FDA) - 21 CFR Part 820, International Organization for Standardization (ISO) - ISO 9001, ISO 13485, Pharmaceuticals and Medical Devices Agency (PMDA), Therapeutic Goods Administration (TGA), China Food and Drug Administration (CFDA), and others.
User-Friendly Interface
Our system design makes it easy for users to accomplish their goals and complete tasks, while minimizing errors and confusion. It considers the needs of various user groups, and provides clear and helpful feedback throughout the user journey. Qualityze strives to create an emotional connection with the user, resulting in increased satisfaction, loyalty, and a positive brand image.
The Start of Something Amazing.
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
- Gain a deeper understanding of changing customer needs.
- Establish long-term best practices aligned with their objectives and goals.
- Define and streamline operational workflows and regulations.
- Strive for excellence in business performance.
Why Change Management Is Important?
Change management is an essential process that should be integrated into different functions like document management, risk management, design control, and new product development. This approach enables organizations to grow and progress efficiently. It also encourages organizations to:
How to establish Organizational Best Practices for Changing Needs to Retain Leadership in Market?
Qualityze Change Control Management Software meets the evolving demands of businesses. It enhances adaptability and prepares for unforeseen situations. Drive organizational excellence by improving workforce understanding of customer needs. Our solution ensures employees contribute to business goals and meet end-user expectations. Boost productivity and meet customer needs with Qualityze Change Management Software. Request a product demonstration for more information. You can contact us through our website for prompt assistance.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
Company
© 2025 Qualityze | All rights reserved. | Privacy Policy

















