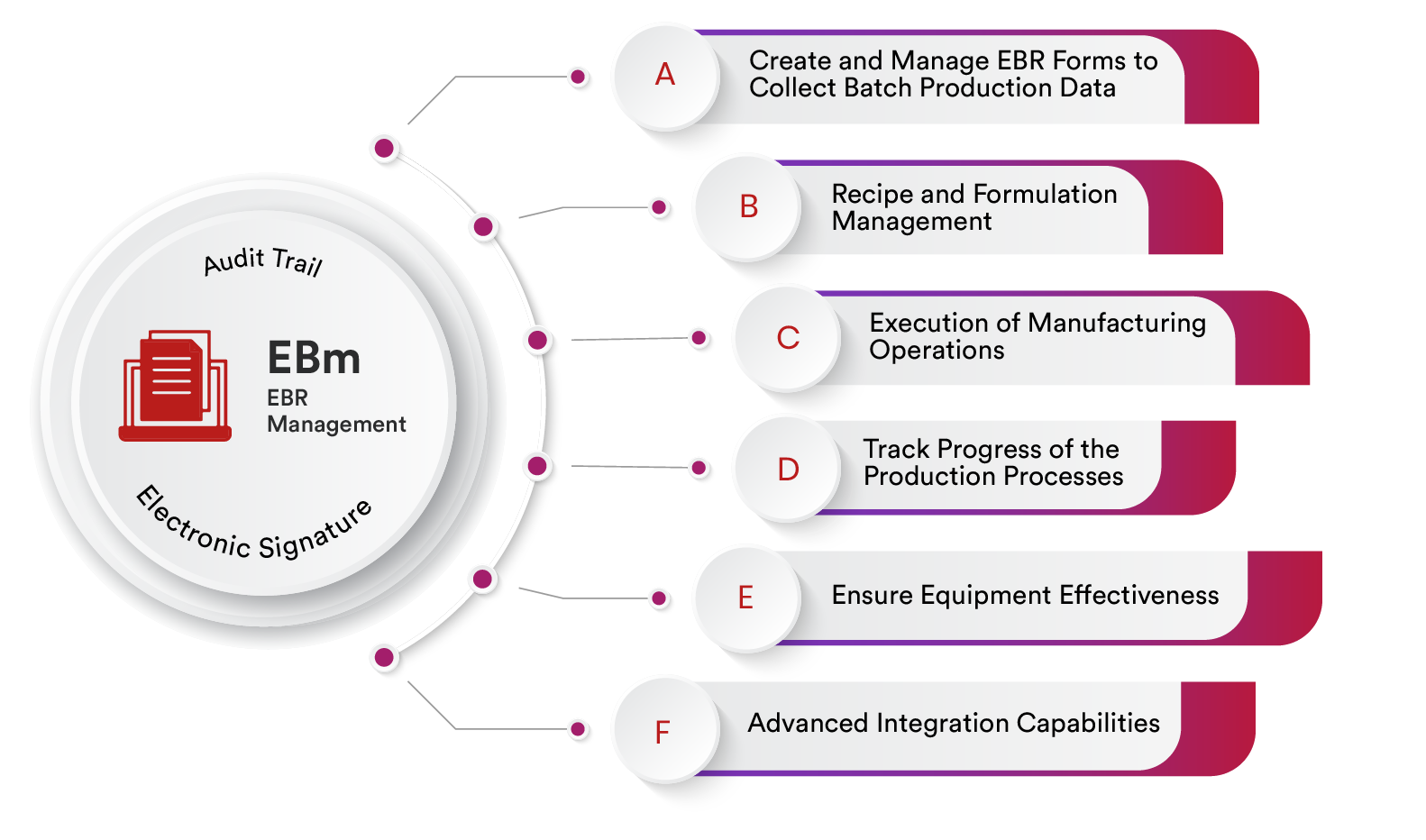
EBR Management
Precision in Every Batch, Perfection in Every Record
In the fast-paced world of manufacturing, especially in regulated industries, ensuring proper product handling and quality control throughout the production stages is paramount. Regulatory bodies like the FDA (Food and Drug Administration) play a pivotal role in maintaining the integrity and safety of products. They accomplish this by enforcing stringent regulations and Good Manufacturing Practices (GMP) to guarantee consistent, high-quality products for consumers. One indispensable aspect of adhering to these regulations is the preparation and retention of electronic batch records, containing comprehensive data on materials, dates, equipment, analysis, labeling, and more. These records provide a crucial element: TRACEABILITY.
Unlock Manufacturing Excellence: Qualityze Electronic Batch Record Management Software
Qualityze Electronic Batch Record Management system is a state-of-the-art, cloud-based solution for precise batch record management. Our innovative platform allows manufacturers to harness the capabilities of cutting-edge technology, resulting in the detection of errors, improved operational efficiency, cost reduction, and the attainment of global scalability. With Qualityze ability to automate the collection of critical data, you can move away from the traditional paper-based record-keeping practices. By doing so, it not only saves valuable time for your organization, but also drastically reduces the risk of errors that can occur during manual data entry.

Create and Manage EBR Forms to Collect Batch Production Data
Achieving consistent and accurate collection of batch process data is essential in today's manufacturing landscape. Qualityze understands that different businesses have unique requirements when it comes to data collection. With this understanding Qualityze EBR Software provides configurable forms and data fields tailored to your specific business and compliance needs. With Qualityze you get a streamlined approach to data management with leads to improved operational efficiency by reducing the time spent on data entry, retrieval, and validation, allowing your team to focus on more value-added tasks.

Recipe and Formulation Management
One of the key process steps in Electronic Batch Record (EBR) Software is Recipe and Formulation Management. Qualityze EBR Management Software facilitates the creation and management of standardized recipes as digital templates, ensuring that each batch of the product is produced consistently, adhering to specified ingredient proportions. In addition, Qualityze Electronic Batch Records Management System provides a user-friendly interface that guides users through the formulation process with precision to ensure that the correct ingredients and quantities are used, reducing the risk of formulation errors. This is especially crucial in industries where even minor deviations in ingredient proportions can significantly impact product quality and compliance.

Execution of Manufacturing Operations
Qualityze Electronic Batch Record (EBR) systems simplifies the execution of manufacturing operations allowing you to predefined manufacturing tasks such as mixing, blending, heating, and cooling with meticulous precision. With Qualityze EBR systems you can ensure that each task is executed according to the prescribed instructions. With its capability to provide users with step by step instructions and guided workflow by the system, minimizes the risk of errors or deviations. These steps include specifying the order of tasks, required materials, equipment settings, and quality checks. With Qualityze EBR systems, there's no room for ambiguity; everything is spelled out clearly.

Track Progress of the Production Processes
In the dynamic world of manufacturing, the ability to track and manage the progress of production processes is crucial for ensuring efficiency, quality, and compliance. With Qualityze, you can monitor production processes comprehensively, right from the creation of the order form to the final verification stage. This end-to-end visibility ensures that you can maintain quality control at every step of the production journey. With built in functionality like alerts, notifications, you can track every action and data point, enabling you to identify issues before they become major problems, saving both time and resources.

Ensure Equipment Effectiveness
Smart manufacturing companies follow internal processes, standards, and external regulations to optimize production and minimize downtime. With seamless integration with Qualityze Calibration and Maintenance Management system, you can proactively manage the manufacturing process confirming that each piece of equipment and tool is operating within optimal performance parameters. With built in intelligence of Qualityze EBR software, it not only monitors equipment performance but also has the capacity to alert or prevent equipment usage in the manufacturing process if it falls within a predefined range for calibration or maintenance schedules. This intelligence empowers you to make well-informed decisions, minimizing risks and optimizing production.

Advanced Integration Capabilities
Qualityze EBR Management offers advanced integration capabilities, ensuring that you have complete control over your batch records and manufacturing processes. Qualityze integrates with a range of other Qualityze systems, including the CAPA (Corrective and Preventive Actions) management system, Document Management System, Training Management system, Nonconformance Management System, Deviation management system, Inspection Management system and more. Additionally, Qualityze EBR can also integrated with third party systems like ERP, Inventory management systems to create a cohesive ecosystem that streamlines the entire manufacturing operations.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
Qualityze Electronic Batch Record (EBR) Management Software is designed to help you reduce the Cost of Quality associated with batch record management. It streamlines and automates processes, leading to significant cost savings. By leveraging advanced features and capabilities, Qualityze EBR Management ensures efficient and error-free operations.
Key benefits:
- Streamlined and automated batch record management processes for cost savings.
- Elimination of manual errors and inefficiencies, reducing rework and associated costs.
- Real-time visibility into batch records, enabling proactive decision-making and issue resolution.
- Improved compliance with regulatory requirements, avoiding costly penalties.
- Enhanced productivity and operational efficiency, resulting in cost savings.
With Qualityze EBR Management Software, you can optimize your batch record management processes, achieve higher quality standards, and realize substantial cost savings in your operations.
Recognitions



EBR management benefits to various operational functions
Every business function has unique expectations from a solution, find out what Qualityze can deliver from your functions perspective.
Quality Assurance
Electronic Batch Record Management Software plays a crucial role in quality assurance by automating and streamlining batch record processes, ensuring compliance, reducing errors, enhancing visibility, and driving operational efficiency.
Risk Management
Electronic Batch Record Management plays a crucial role in risk management by providing standardized processes, real-time visibility, and traceability, enabling proactive identification and mitigation of risks associated with batch production and compliance.
Process Improvement
Electronic Batch Record Management Software plays a vital role in process improvement by automating workflows, reducing errors, providing real-time visibility, and enabling data-driven decisions for enhanced efficiency and quality in batch record management processes.
Supply Chain Management
EBR Management plays a crucial role in supply chain management by providing real-time visibility, streamlining processes, ensuring compliance, and optimizing efficiency, leading to improved inventory management and overall supply chain performance.
Production Management
Electronic Batch Record Management plays a crucial role in production management by automating workflows, ensuring compliance, reducing errors, and providing real-time visibility into batch records for streamlined and efficient production processes.
Compliance and Regulatory Affairs
Electronic Batch Record Management plays a vital role in compliance and regulatory affairs by providing standardized processes, accurate documentation, real-time visibility, and audit trails, ensuring adherence to regulatory requirements and facilitating efficient inspections.
Experience Qualityze Difference in managing Quality
We empower organizations to uphold exceptional quality standards while providing the following advantages:
Configurable Cloud-Based Platform
Our system is developed on the world's foremost cloud-based platform, Salesforce.com, which allows businesses to effortlessly personalize it to their exact requirements. It is an adaptable and scalable system that expands in tandem with the growth of your organization. Its wide-ranging configurability broadens the functional scope of an EQMS to an infinite array of creative applications, making it an extremely valuable solution for your organization.
Compliance Ready
Our system adheres to compliance standards for every industry and follows the relevant regulations set by regulatory committees, such as the Food and Drug Administration (FDA) - 21 CFR Part 820, International Organization for Standardization (ISO) - ISO 9001, ISO 13485, Pharmaceuticals and Medical Devices Agency (PMDA), Therapeutic Goods Administration (TGA), China Food and Drug Administration (CFDA), and others.
User-Friendly Interface
Our system design makes it easy for users to accomplish their goals and complete tasks, while minimizing errors and confusion. It considers the needs of various user groups, and provides clear and helpful feedback throughout the user journey. Qualityze strives to create an emotional connection with the user, resulting in increased satisfaction, loyalty, and a positive brand image.
The Start of Something Amazing.
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
- Enhanced inventory and resource management, resulting in reduced material losses.
- Greater control over batch data and processes.
- Reduced production cycle time and compliance costs.
- Fewer deviations and production errors.
- Faster resolution and response management.
- Improved productivity and scalability.
- Enhanced bottom line.
Why EBR Management Is Important?
In most cases, enterprises utilize batch manufacturing processes to produce their products. These processes can be customized to suit the manufacturer's requirements and ensure quality control. In batch processes, everything is well-organized and documented. Consequently, the electronic batch record becomes even more effective in reducing errors, ensuring compliance, and enhancing efficiency. Additionally, digitizing your batch records can bring numerous benefits, such as:
Does Qualityze EBR Management Gain Real-Time Production Visibility to Ensure Quality from Day One?
Qualityze EBR Management software ensures quality from day one by streamlining production batch record management. Its user-friendly interface simplifies the setup of master batch record documents, while digital signatures validate data at every stage. Integration with enterprise quality systems allows for material inspections, training, verification, audits, and reconciliations, ensuring compliance, process analysis, and efficiency. Leveraging best practices and cloud technology, the software provides real-time visibility into production trends, enabling informed decision-making and reducing deviations. With a centralized repository for all EBR records, teams can track and improve performance, leading to enhanced profitability and bottom-line impact.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
Company
© 2025 Qualityze | All rights reserved. | Privacy Policy

















