
How to Mitigate Manufacturing Industry Hazards with QMS
If you are on a manufacturing floor long enough, you will eventually figure out a problem with the hazards that they are not always loud or dramatic. Quietly they are there—almost invisible—until one unusual shift; one overlooked detail, or one rushe...

How to Avoid FDA Warning Letters by Implementing an EQMS
Sometimes the distance between a smooth audit and an FDA warning letter is just one overlooked document, one outdated SOP, or one missing signature. Organizations rarely see it coming, until the letter arrives, public, permanent, and costly. In an er...

How QMS Drives Quality Improvement and Regulatory Compliance
Ready for a real talk moment? We often see compliance as a necessary evil that comes with a mountain of rules we have to climb just to stay in business. It's boring, it's tedious, and we treat it like a totally separate chore. But honestly, that min...

QSR vs QMSR: The Best Guide for Med Device Manufacturers
Why quality systems matter in medical device manufacturing
In the medical industry, "quality" is the only thing standing between a patient and a preventable tragedy. A robust quality system isn't just about passing an inspection; it's about ensur...

How To Integrate QMS And CTMS For Improved Operations
Clinical trials demand both operational efficiency and rigorous quality assurance. The friction caused by managing these objectives in separate software platforms poses a significant threat to timelines and compliance. Recognizing this operational di...

Salesforce vs AWS vs Azure - The Better Platform for EHS
In today's mission-critical regulatory environment, Environmental, Health, and Safety (EHS) is a strategic imperative. Organizations are moving beyond fragmented spreadsheets, demanding scalable, modern Cloud EHS software that can handle everything f...

How To Strengthen Workplace Safety with a Modern PTW System
In high-hazard industries, the paper trail of the traditional Permits to Work is often a liability waiting to happen—not a safety net. The shift from manual, error-prone processes to a robust, modern digital PTW system has now become imperative rathe...

Top Industrial Safety Hazards and How to Eliminate Them
Industrial environments in the present are fast and complex in nature, and they also combine technical machines with very demanding production schedules. All these factors have the effect that these hazards can appear very fast and can develop furthe...

Top QMS Tools You Need to Improve Your Quality Processes
The margin for error in regulated industries has evaporated. Without robust QMS Tools, organizations invite financial penalties, brand reputation damage, and product recalls. If your quality team is drowning in a sea of spreadsheets, binders, and sys...

How to Choose The Best Customer Complaint Management Software
Deciding on the best customer complaint management software to manage your customer complaints is not just about adding another tool to your technological resources. Essentially, it is about extending the whole customer experience cycle. Customers no...

How To Design QMS Workflow for Life Sciences Company?
Designing a QMS workflow for a life sciences company is a little-like designing a circulatory system—every process, every approval; every document flows through it. When it works well, the organization feels healthy and efficient. It slows down t...

How BPM And QMS Integration Adds Value to an Organization
Companies nowadays are heavily pressed by various factors—more stringent rules, quicker market demands, decreasing profits, and increasing complexity of worldwide operations. In the middle of all these, there are still two systems which run in a way ...

Why Data-Driven Quality Management Is the Only Way Forward
Quality management, which is the main topic of discussion, has thrown a curve to the majority of the organizations by changing rapidly instead of gradually as they have anticipated. It is no longer a recorded-heavy, slow-function but has turned into ...

What FDA, EMA, and ISO Say About AI in Quality Systems
AI is revolutionizing life sciences and has a major impact on how medical devices and pharmaceutical companies manage quality. As a result, top regulators like FDA, EMA, and ISO cannot be indifferent to such a powerful change in quality systems.
...

Industry 4.0: Redefining Pharma Quality Through Digital Tech
The pharmaceutical industry is undergoing its most profound transformation driven by the need for speed, personalized medicine, and intense global regulatory scrutiny. Traditional, paper-based quality systems are turning out to be a critical bottlene...

Safety 4.0: Unlock Smarter Ways to Prevent Workplace Risks
The industrial world has always evolved its approach to safety, moving from basic compliance to complex systems-based management. However, in today’s hyper-connected environment, traditional, paper-based, or siloed safety programs are critically inad...

The Role of Goods Received Note (GRN) in RMA
When a customer returns a product, most people only see the surface: a package shows up at the warehouse, someone signs for it, and the refund or replacement happens. But behind the scenes, there’s an organized process that keeps everything running s...

How to Automate Supplier Qualification Process with a EQMS?
Maintaining an Approved Supplier List (ASL) Management system that is both compliant and efficient requires digital transformation. Discover how to Automate Supplier Qualification Process seamlessly.
The modern global supply chain introduces un...

How Integrating QMS with ERP, LMS, and LIMS Can Boost Growth
For too long, Quality has been treated as a departmental function—a cost center operating in its own silo. Today, leading enterprises are recognizing that quality is a strategy, not a department, and the path to genuine, sustained growth runs directl...

What is Quality Management and How Does It Work?
The pressure for operational excellence in modern businesses has never been greater. Customers demand perfection, and regulatory bodies enforce the harshest of penalties for non-adherence. It is this demanding environment that makes Quality Managemen...

What Is A Permit To Work System? Why It Is Important?
Operational settings, especially in regulated industries like life sciences and advanced manufacturing, entail tasks that are intrinsically highly risky. These activities, ranging from essential electrical maintenance to the execution of a hot work p...

EHS 4.0: Discover How It’s Transforming Quality and Safety
The pressure to demonstrate both GxP compliance and environmental stewardship has never been higher, necessitating a Digital Transformation EHS overhaul.
Industry 4.0 is here, and it is time that safety and quality systems keep pace with the mo...

Return Merchandise Authorization (RMA): What You Need to Know
It is inevitable for every company to deal with a return at some point in time. A customer might have a product that didn't work as intended, was delivered damaged, or was just not the right fit. The simple-looking request, however, hides a complic...

How to Automate Vendor Qualification Process with an EQMS?
The pressure on quality teams to ensure supplier adherence to complex, evolving GxP and ISO standards has reached a critical point. Imagine eliminating weeks of paper shuffling, chasing signatures, and inconsistent scoring—that's the power of automat...

Cost of Non-Conformance in Life Sciences that You Need to Know
The life sciences industry is a sector where winning equates to being exact, safe, and following the rules. It is an area where errors, even the tiniest ones, are very costly. Non-conformance is not just a matter of increased documentation in the cas...

Supplier Collaboration Strategies & Benefits You Need to Know
In life sciences and manufacturing, the integrity of your final product is only as strong as your most vulnerable external link—your weakest point of Digital Supplier Oversight.
Today's global supply chain is intensely complex, burdened by regul...
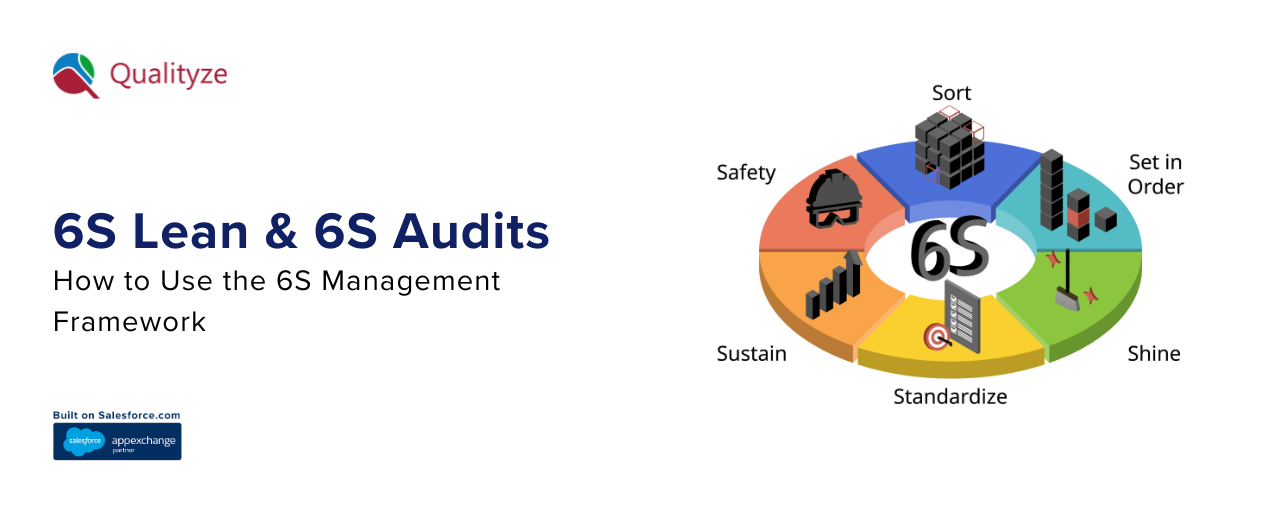
6S Lean & 6S Audits: How to Use the 6S Management Framework
The 6S framework basically consists of six simple yet effective ideas: Sort (Seiri), Set in Order (Seiton), Shine (Seiso), Standardize (Seiketsu), Sustain (Shitsuke), and Safety (the sixth "S"). If it is used properly, it changes messy working areas ...

What Is Digital Watermarking and Why It Is Important in QMS
In modern quality management, the integrity and security of documentation are paramount, especially for regulated industries. As organizations transition to digital processes, the vulnerability of Electronic Record Management to unauthorized access o...

Supplier Performance Management (SPM): How to Optimize It?
In today’s hyper-connected global marketplace, the effective management of vendor relationships—known as Supplier Performance Management (SPM)—is no longer a procurement of luxury, but a non-negotiable quality imperative. For manufacturers in highly ...

How To Design QMS Workflow For Manufacturing Company?
Digital transformation has hit the factory floor, yet many quality systems lag behind. Learn how expert QMS Workflow Design for Manufacturing can bridge this gap and turn compliance into a competitive edge.
In the demanding world of production, ...





























